Page 12 - PHESGO (PERTUZUMAB-TRASTUZUMAB) - Product Monograph
P. 12
Primary endpoint
Non-inferiority of the Cycle 7 (i.e., pre-dose Cycle 8) pertuzumab serum Ctrough.
Secondary endpoints
Non-inferiority of the Cycle 7 (i.e., pre-dose Cycle 8) trastuzumab Ctrough, efficacy
(pCR), and safety.
‡
Stratification factors
Hormone receptor status; clinical stage at presentation (Stage II-IIIA or IIIB-IIIC);
type of chemotherapy.
*Fixed-dose SC combination of trastuzumab and pertuzumab dosing: 1200 mg pertuzumab/600 mg trastuzumab/30,000 units
hyaluronidase loading dose, followed by 600 mg pertuzumab/600 mg trastuzumab/20,000 units hyaluronidase maintenance
dose.
®
† IV PERJETA dosing: 840 mg loading dose, 420 mg for subsequent cycles; IV trastuzumab dosing: 8 mg/kg loading dose, 6
mg/kg for subsequent cycles. In adjuvant period, substitution of IV trastuzumab for SC trastuzumab (trastuzumab-oysk) was
permitted at investigator discretion. Trastuzumab-oysk was given as a fixed dose of 600 mg. 61 patients received trastuzumab-oysk.
‡ pCR=pathological complete response (ypT0/is, ypN0, defined as the absence of invasive neoplastic cells in the breast and in the
axillary lymph nodes).
Chemotherapy regimens: ddAC dosing: doxorubicin (60 mg/m ) and cyclophosphamide (600 mg/m ) every 2 weeks; AC dosing:
2
2
2
2
doxorubicin (60 mg/m ) and cyclophosphamide (600 mg/m ) every 3 weeks; paclitaxel dosing: 80 mg/m weekly; docetaxel
2
dosing: 75 mg/m every 3 weeks. Docetaxel dose could be escalated to 100 mg/m at subsequent cycles at investigator’s
2
2
discretion.
AC=doxorubicin + cyclophosphamide; ddAC=dose-dense doxorubicin-cyclophosphamide.
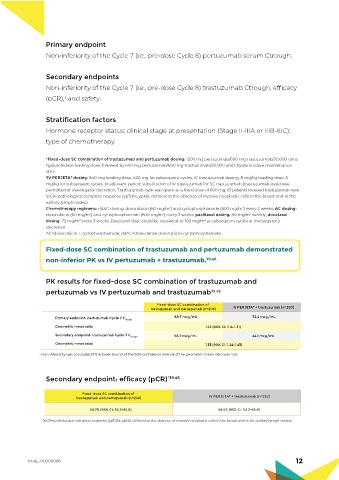
Fixed-dose SC combination of trastuzumab and pertuzumab demonstrated
non-inferior PK vs IV pertuzumab + trastuzumab. 39,46
PK results for fixed-dose SC combination of trastuzumab and
pertuzumab vs IV pertuzumab and trastuzumab 39,46
Fixed-dose SC combination of
trastuzumab and pertuzumab (n=206) IV PERJETA ® + trastuzumab (n=203)
88.7 mcg/mL 72.4 mcg/mL
Primary endpoint: pertuzumab Cycle 7 C trough
Geometric mean ratio 1.22 (90% CI: 1.14-1.31)
Secondary endpoint: trastuzumab Cycle 7 C trough 58.7 mcg/mL 44.1 mcg/mL
Geometric mean ratio 1.33 (90% CI: 1.24-1.43)
• Non-inferiority was concluded if the lower bound of the 90% confidence interval of the geometric mean ratio was ≥0.8
Secondary endpoint: efficacy (pCR) *39,46
Fixed-dose SC combination of
®
trastuzumab and pertuzumab (n=248) IV PERJETA + trastuzumab (n=252)
59.7% (95% CI: 53.3-65.8) 59.5% (95% CI: 53.2-65.6)
*pCR=pathological complete response (ypT0/is, ypN0, defined as the absence of invasive neoplastic cells in the breast and in the axillary lymph nodes).
M-AE-00000086 12