Page 14 - PHESGO (PERTUZUMAB-TRASTUZUMAB) - Product Monograph
P. 14
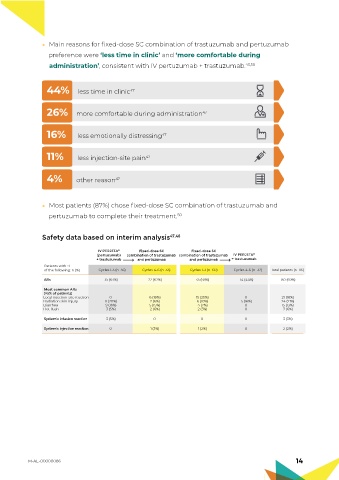
Main reasons for fixed-dose SC combination of trastuzumab and pertuzumab
preference were ‘less time in clinic’ and ‘more comfortable during
administration’, consistent with IV pertuzumab + trastuzumab. 43,50
44% less time in clinic 47
26% more comfortable during administration 47
16% less emotionally distressing 47
11% less injection-site pain 47
4% other reason 47
Most patients (87%) chose fixed-dose SC combination of trastuzumab and
pertuzumab to complete their treatment. 50
Safety data based on interim analysis 47,48
IV PERJETA ® Fixed-dose SC Fixed-dose SC
(pertuzumab) combination of trastuzumab combination of trastuzumab IV PERJETA ®
+ trastuzumab and pertuzumab and pertuzumab + trastuzumab
Patients with ≥1
of the following: n (%) Cycles 1-3 (n=56) Cycles 4-6 (n=33) Cycles 1-3 (n=60) Cycles 4-6 (n=32) Total patients (n=116)
ARs 35 (63%) 22 (67%) 35 (58%) 14 (44%) 80 (69%)
Most common ARs
(>5% of patients)
Local injection-site reaction 0 6 (18%) 15 (25%) 0 21 (18%)
Radiation skin injury 11 (20%) 2 (6%) 6 (10%) 5 (16%) 24 (21%)
Diarrhea 9 (16%) 5 (15%) 4 (7%) 0 15 (13%)
Hot flush 3 (5%) 2 (6%) 2 (3%) 0 7 (6%)
Systemic infusion reaction 3 (5%) 0 0 0 3 (3%)
Systemic injection reaction 0 1 (3%) 1 (2%) 0 2 (2%)
M-AE-00000086 14