Page 13 - PHESGO (PERTUZUMAB-TRASTUZUMAB) - Product Monograph
P. 13
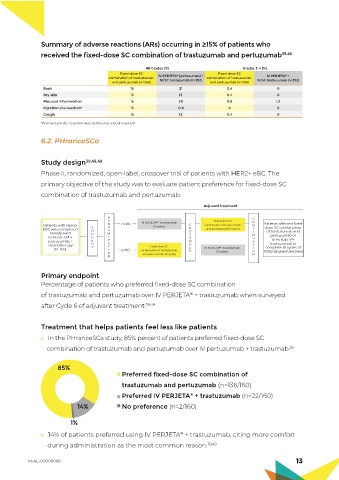
Summary of adverse reactions (ARs) occurring in ≥15% of patients who
received the fixed-dose SC combination of trastuzumab and pertuzumab 39,46
All Grades (%) Grades 3-4 (%)
Fixed-dose SC Fixed-dose SC
combination of trastuzumab IV PERJETA ® (pertuzumab) + combination of trastuzumab IV PERJETA ® +
and pertuzumab (n=248) IV/SC trastuzumab (n=252) and pertuzumab (n=248) IV/SC trastuzumab (n=252)
Rash 16 21 0.4 0
Dry skin 15 13 0.4 0
Mucosal inflammation 15 20 0.8 1.2
Injection site reaction* 15 0.8 0 0
Cough 15 13 0.4 0
*An injection site reaction was defined as a local reaction.
6.2. PHranceSCa
Study design 39,48,49
Phase II, randomized, open-label, crossover trial of patients with HER2+ eBC. The
primary objective of the study was to evaluate patient preference for fixed-dose SC
combination of trastuzumab and pertuzumab.
Adjuvant treatment
R C
A IV PERJETA ® + trastuzumab Fixed-dose SC O
Patients with HER2+ S N D n=80 (3 cycles) C combination of trastuzumab N Patients selected fixed-
dose SC combination
EBC who completed U O R O and pertuzumab (3 cycles) T I of trastuzumab and
neoadjuvant R M S N
IV PERJETA ® + G I S U pertuzumab or
trastuzumab + E Z A O A IV PERJETA ® +
trastuzumab to
chemotherapy R Y T Fixed-dose SC V E T complete 18 cycles of
(N=160) I n=80 combination of trastuzumab R IV PERJETA ® + trastuzumab I
O and pertuzumab (3 cycles) (3 cycles) O N HER2-targeted treatment
N
Primary endpoint
Percentage of patients who preferred fixed-dose SC combination
of trastuzumab and pertuzumab over IV PERJETA + trastuzumab when surveyed
®
after Cycle 6 of adjuvant treatment. 39,49
Treatment that helps patients feel less like patients
In the PHranceSCa study, 85% percent of patients preferred fixed-dose SC
combination of trastuzumab and pertuzumab over IV pertuzumab + trastuzumab. 39
85%
Preferred fixed-dose SC combination of
trastuzumab and pertuzumab (n=136/160)
Preferred IV PERJETA + trastuzumab (n=22/160)
®
14% No preference (n=2/160)
1%
14% of patients preferred using IV PERJETA + trastuzumab, citing more comfort
®
during administration as the most common reason. 39,49
M-AE-00000086 13